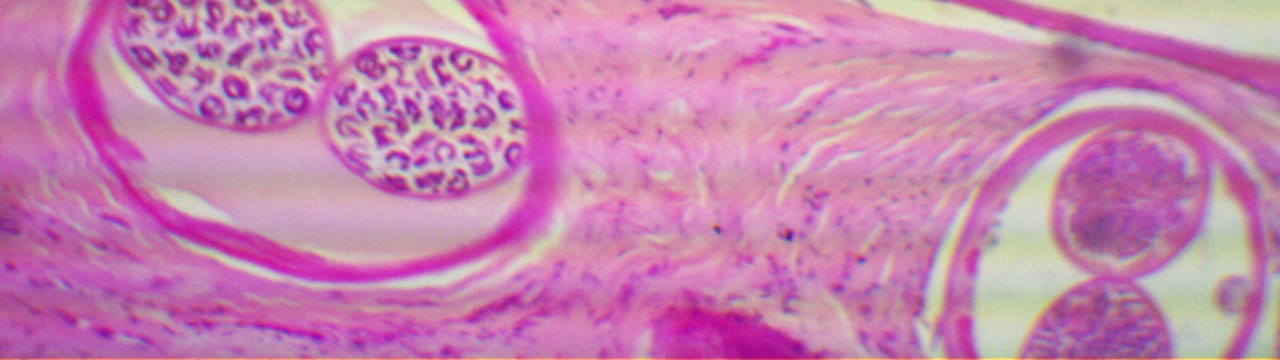
Research in the laboratory is focused on the control and pathology of nematode infections. We have developed a vaccine against Onchocerca volvulus, the causative agent of river blindness, and our current research emphasis is on the mechanisms of protective immunity induced by the vaccine in mice. Research on the nematode Strongyloides stercoralis is directed at determining the mechanism and therapy of hyperinfection caused by this infection.
Abraham Research
Bluemle Life Sciences Building
233 South 10th Street, Suite 530
Philadelphia, PA 19107
Research Projects
Development of a vaccine against Onchocerca volvulus, the causative agent of river blindness
The initial focus of this project was to identify the optimal formulation of a recombinant vaccine to protect humans from infection with the nematode O. volvulus. We tested adjuvants and antigen combinations to identify the vaccine with highest efficacy in a mouse model. Current research is directed at identifying the mechanism of the protective immune response induced by the vaccine with an emphasis on antibody and cell interactions. In addition, Collaborative Cross mice are being used to test the vaccine in genetically diverse mice to determine the efficacy of the vaccine in heterogeneous populations as would be seen in humans in endemic regions.
Development of humanized mouse models for Onchocerca volvulus
NSG and humanized NSG mice were tested for their susceptibility to infection with the nematode O. volvulus. We determined that these mice were susceptible to the infection and supported parasite survival, growth and development for at least 12 weeks. Infected humanized mice allowed the identification of unique parasite biomarkers in blood and urine with potential use in diagnosing human infections.
Mechanism and therapeutic development for hyperinfection by Strongyloides stercoralis
Humans infected with S. stercoralis routinely maintain long-term chronic infections, which may evolve into fatal hyperinfection if the patient is treated with steroids or is co-infected with HTLV-1. We have reported that NSG mice are susceptible to the complete life-cycle of S. stercoralis and will develop hyperinfection if exposed to steroids. Further, treatment of mice with dafachronic acid significantly reduced the worm burden in mice undergoing hyperinfection. Current research is directed at determining the mechanism of steroid induced hyperinfection and the interaction between S. stercoralis and HTLV-1 in humanized mice.
