04.03.23
Stress, Obesity & Pancreatic Cancer
By Edyta Zielinska | Illustrations by Ava Schroedl
A common response to cellular stress may be the key to pancreatic cancer in people with obesity, pointing to new therapy for this difficult-to-treat cancer.
Early in her scientific career, as a postdoc working on pancreatic cancer, Elda Grabocka, PhD, read a review article that changed her trajectory. It discussed the importance of cellular stress in cancer formation. A few months later, she discovered that her own line of research intersected with recently discovered stress-related organelles called “stress-granules.” “I had to learn more! I couldn’t help but wonder whether stress granules were the missing link between cancer and obesity,” says Dr. Grabocka.
Obesity, which causes stress and inflammation throughout the body, is a known risk factor for at least 13 types of cancer, but understanding this relationship well enough to block it has been challenging.
Dr. Grabocka knew that stress granules were an unusual sort of cellular compartment. The cell generates these dense globules of RNA and protein in response to cellular stresses like viral infection, neurodegeneration or starvation. In fact, they protect the cell from stress-induced self-destruction. It’s a cellular reflex and defense mechanism that’s present throughout the animal and plant kingdoms. Even tomato plants produce stress granules.
Dr. Grabocka started by studying the link between pancreatic cancer and stress granules, and was able to show stress granules were abundant in the tumors of patients with pancreatic cancer, proving there was a relationship worth exploring.
Indeed, after Dr. Grabocka’s paper on pancreatic cancer published, other researchers showed that many cancers produce high levels of stress granules to prevent their own self-destruction.
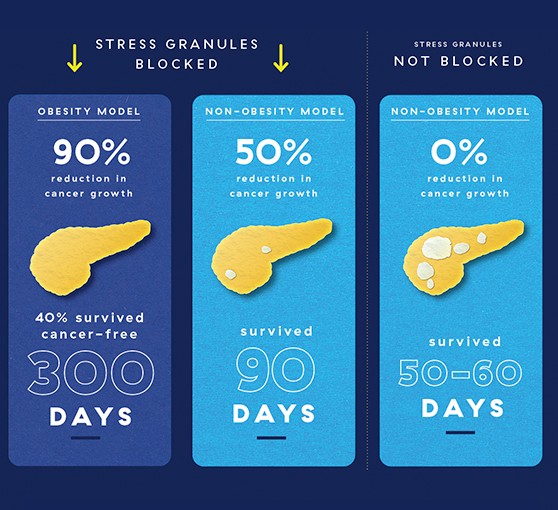
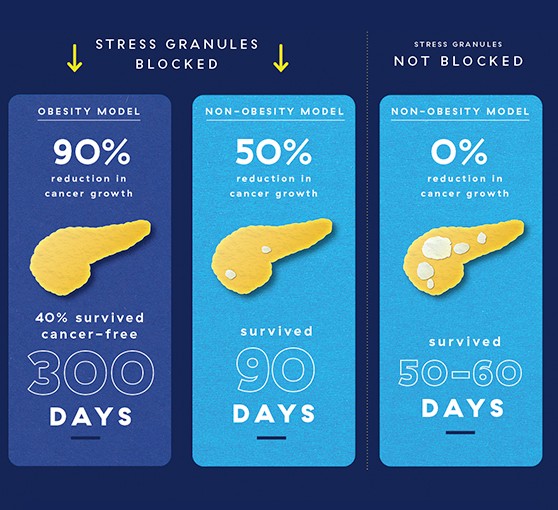
When Dr. Grabocka came to the Sidney Kimmel Cancer Center–Jefferson Health, she began to probe the relationship further. She created a mouse model of pancreatic cancer that blocked the formation of stress granules in cancer cells. Her team saw a whopping 50% reduction in cancer growth in cancer growth in those mice. This was already an impressive effect, but Dr. Grabocka wondered whether it could be even bigger in obesity-related pancreatic cancer.
Obesity affects two thirds of all adults in the U.S. and 50% globally. It also doubles the risk and mortality for pancreatic cancer. About 33% of pancreatic cancer is obesity-related, a number that is only expected to rise in the coming decades.
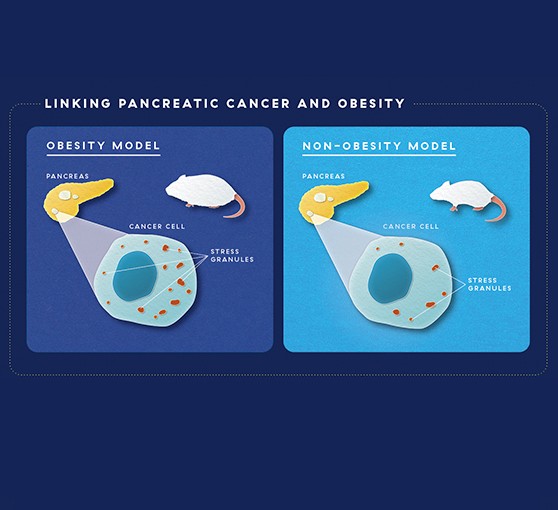
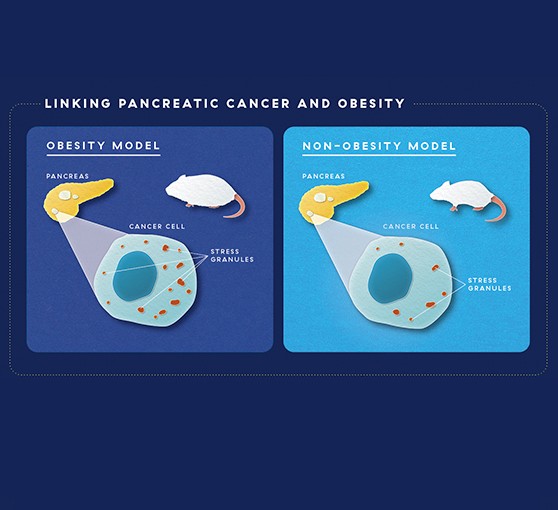
To test the role of obesity, the researchers took two different types of mouse models of obesity and looked at pancreatic cancer in these mice. Both obesity models had five to eight times the amount of stress granules in their cancers as non-obese mice. This suggested that the cancers in obese mice might be dependent on stress-granules for their growth. “When we take away the thing a cancer depends on to live, we can kill the cancer,” says Dr. Grabocka.
When the researchers blocked stress-granule formation in obese mice with pancreatic cancer, the results were really quite surprising. They either saw no cancer growth, or 1/14 and 1/20 the amount of growth they’d expect in obese mice with intact stress granules.
The most striking difference was their overall survival. Normally in models of pancreatic cancer, mice die very quickly, within 50-60 days. In obese mice whose stress granules were blocked, 40% were cancer-free after 300 days.
These experiments, published in the high-impact journal Cancer Discovery, showed that stress granules were actually driving the growth of cancer at the very start. “This is the first direct evidence linking stress granules to cancer progression,” says Dr. Grabocka.
Importantly, Dr. Grabocka’s lab also identified drug targets that block stress granules in obesity-related pancreatic cancer. The next step is to see if they can be translated for use in humans.
Capitalizing on Stress
To test the role of stress granules in cancer, Dr. Grabocka and colleagues looked at pancreatic cancer in obese vs. non-obese mouse models, where they saw more stress granules in obese mice. Blocking stress granules in obese and non-obese mice led to less cancer growth and increased survival compared to mice whose stress granules were not blocked. But in obese mice, the difference was much greater, suggesting that blocking stress granules in this cancer subtype could offer a new avenue for treatment.